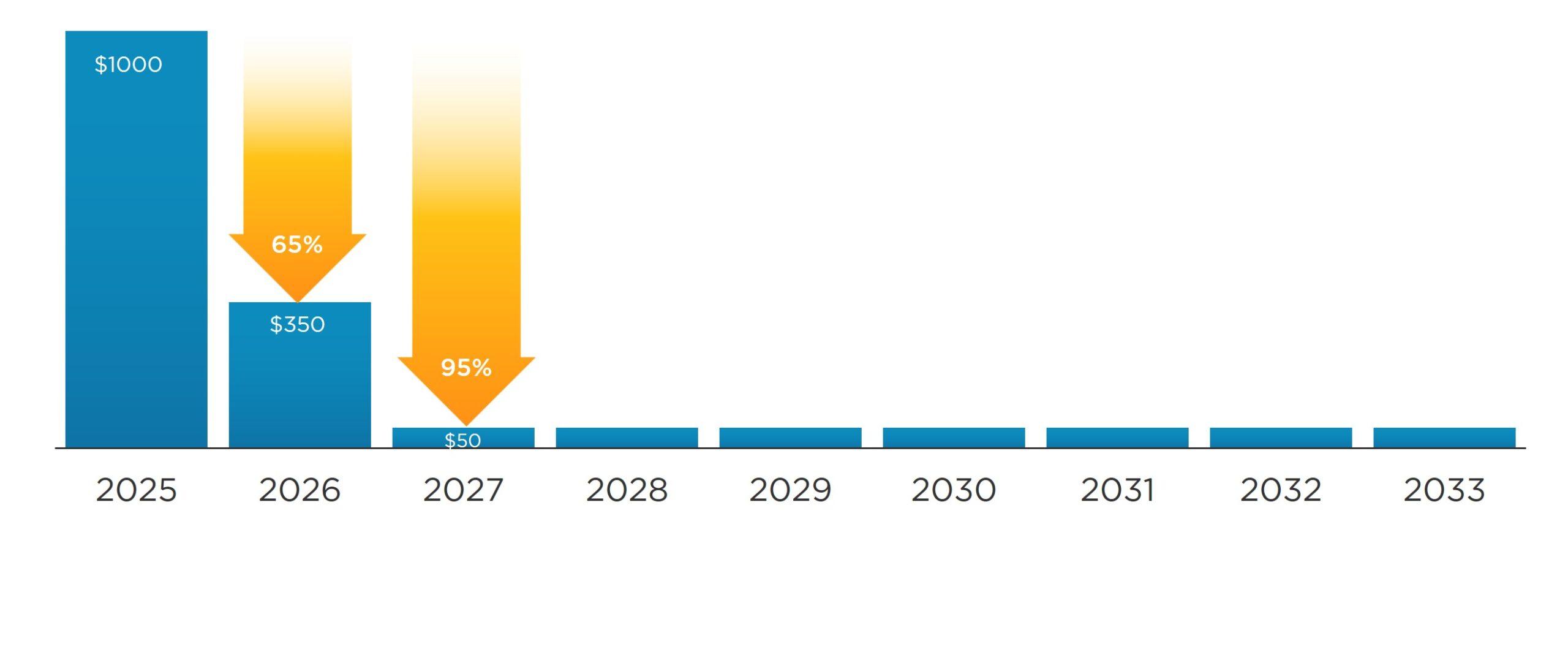
The Increasing Exploitation of Patient Assistance Programs
Copay accumulator programs have only one purpose; increase profits for payers. Patients don’t gain an advantage, nor do manufacturers. On top of that, providers face the difficulty of helping patients